


Copyright © Meta Pharmaceuticals Inc.

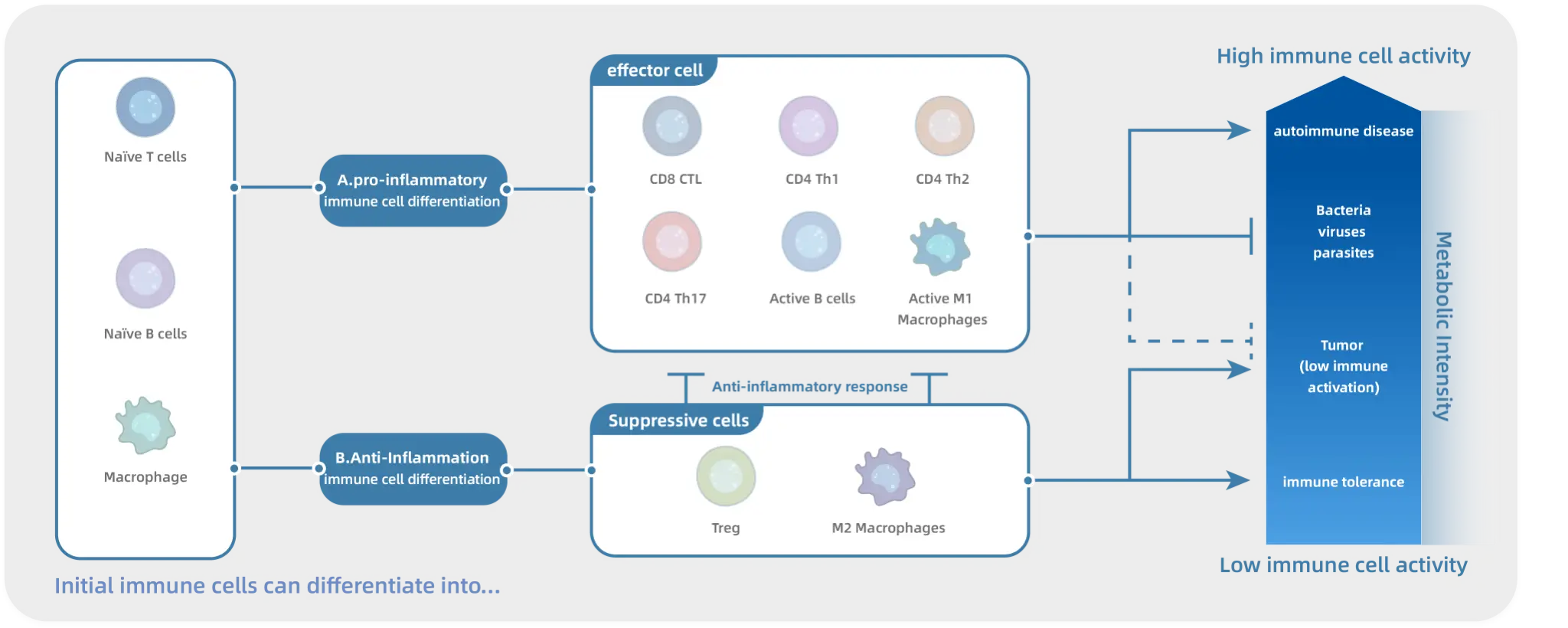
Be a medicine innovator The META team has spent 7 years developing the “Modulated Immunometabolic Pathway Strategy”, a revolutionary approach to treating self-infection diseases. This breakthrough therapy provides a safer, more effective, and novel way of fighting such diseases, greatly enhancing patient outcomes.







META Pharmaceuticals Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease Designation (RPDD) to its investigational new drug META-001-PH for the treatment of primary hyperoxaluria (PH). Primary hyperoxaluria is a rare genetic disorder that can lead to kidney stone formation, renal failure, and can be life-threatening in severe cases. The RPDD is intended to facilitate the development of drugs and biologics for serious and life-threatening rare pediatric diseases that affect fewer than 200,000 people in the U.S. and predominantly occur in patients aged 18 years and younger. This designation is pursuant to section 529(a)(3) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 360ff(a)(3)).
About Primary Hyperoxaluria (PH)
Primary Hyperoxaluria (PH) is an autosomal recessive metabolic disorder in which oxalate is overproduced and deposited in the body due to defects in enzymes responsible for oxalate metabolism in the liver and other organs. Patients typically present with kidney stones, nephrocalcinosis, renal failure, and oxalate deposition in other organs. Severe cases can lead to end-stage renal disease (ESRD) requiring dialysis, kidney transplantation, or combined liver-kidney transplantation. Symptoms of the disease usually appear at the age of 0 to 3. Without intervention, most patients will develop end-stage renal disease during adolescence, which severely threatens their lives. The incidence of PH is estimated to be 1/58,000, affecting more than 10,000 people in the United States and the European Union, and more than 20,000 people in China. Currently, there is no cure for primary hyperoxaluria. Existing treatments are primarily supportive care, including increased fluid intake to dilute oxalate in the urine, and medications such as pyridoxine (vitamin B6) to reduce oxalate production. However, patients’ symptoms and disease progression cannot be effectively controlled.
About META-001-PH
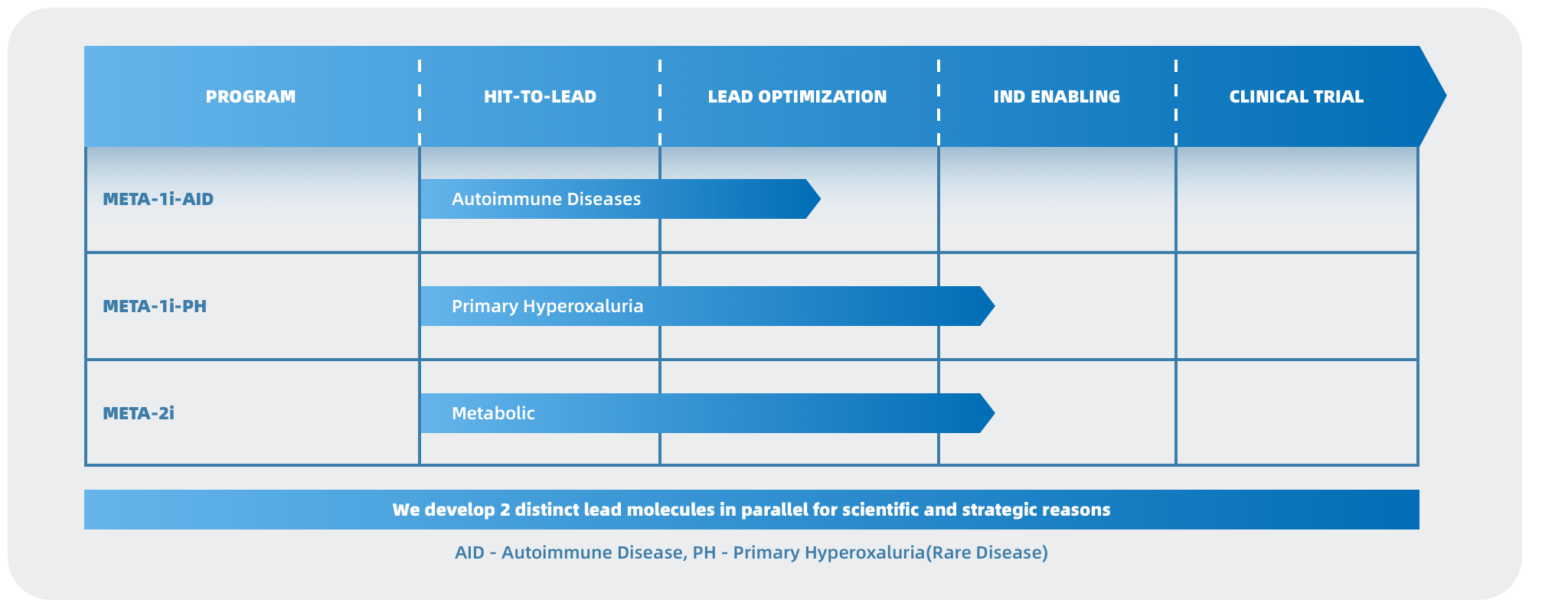
META-001-PH is a groundbreaking small molecule drug developed by META for the treatment of primary hyperoxaluria. This innovative molecule is the product of a collaborative effort between META’s chemistry team and XtalPi’s (2228.HK) AI drug discovery team, who worked together from the initial scaffold screening to the preclinical compound nomination. XtalPi’s automated robotic chemical synthesis lab was responsible for the chemical synthesis of the related molecule series, ensuring a precise and efficient production process. Preclinical experiments in animal disease models have shown that META-001-PH can significantly reduce urinary oxalate excretion by up to 80%. While existing therapeutic agents are unable to effectively control urinary oxalate levels in the long term, META-001-PH, administered orally daily, can maintain oxalate at normal levels, thus demonstrating the potential for better long-term control of kidney stone formation in patients with PH. META-001-PH has also demonstrated good tolerability and safety in preclinical animal models and is undergoing IND-enabling toxicology studies with a clinical Phase I safety assessment on healthy subjects planned for 2025H1 in Australia. This collaboration between META and XtalPi marks a significant advancement in the development of effective treatments for primary hyperoxaluria.
About Rare Pediatric Disease Designation (RPDD)
The Rare Pediatric Disease Designation (RPDD) is an eligibility determination for rare pediatric diseases that affect fewer than 200,000 patients and pose a serious life-threatening risk to children under the age of 18. The RPDD and Priority Review Voucher (PRV) program aims to recognize the significant need for therapies for rare pediatric diseases and to encourage the development of new treatments for these serious or life-threatening conditions. Under the program, sponsors will be eligible to receive a PRV upon approval of a New Drug Application (NDA) or Biologics License Application (BLA) for a rare pediatric disease. PRVs can be used for any subsequent product approval application, reducing the review time by 4-6 months, or can be traded with an average transfer price of more than $100 million in recent years.
About META Pharmaceuticals Inc.
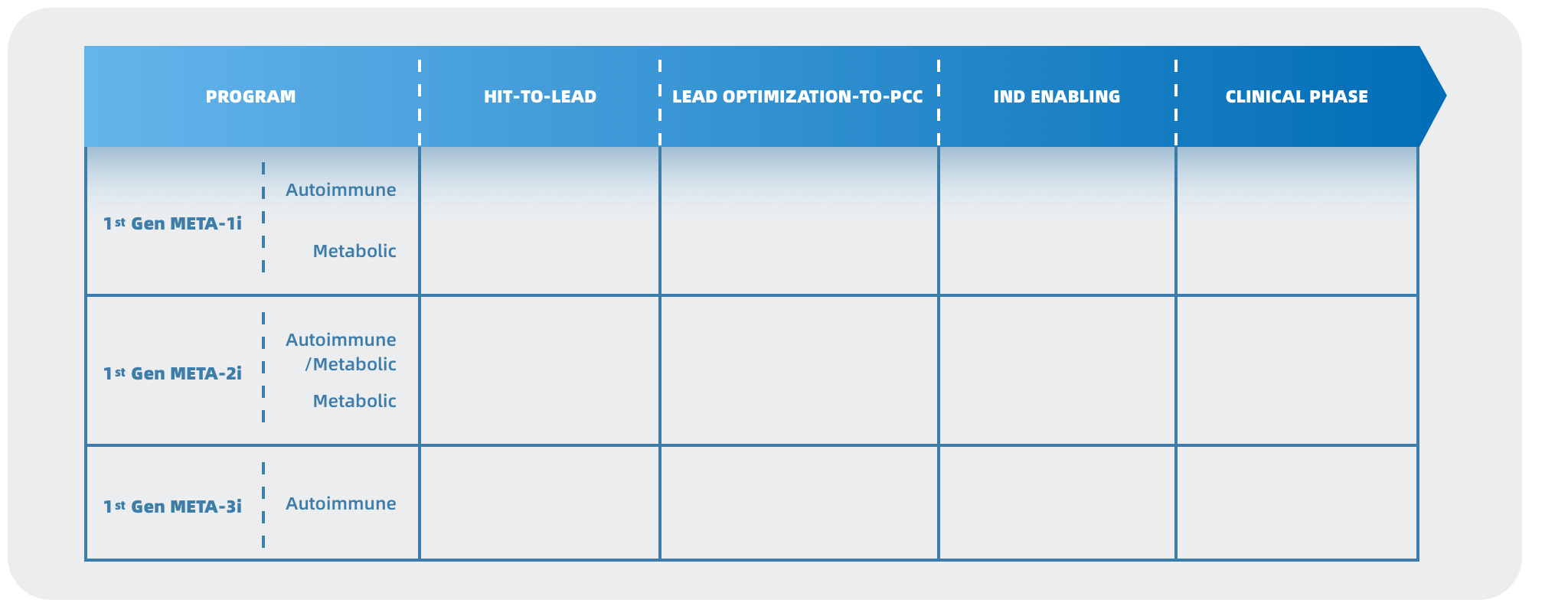
META Pharmaceuticals Inc. (META) is an innovative biopharmaceutical company dedicated to discovering and developing urgently needed breakthrough treatments for a broad range of autoimmune disorders, metabolic diseases, and cancer. As the only company in the Asia Pacific Region leveraging the groundbreaking theory of immuno-metabolism, META leads the way in creating safer and more effective therapies that modulate cellular metabolism to regulate immune system function and other pathways. META has developed two distinct chemical series targeting META-001 for the treatment of autoimmune diseases and primary hyperoxaluria, respectively, which hold the potential to become the next-generation oral treatments for these conditions, addressing significant unmet clinical needs. META Pharmaceuticals Inc. is jointly incubated by XtalPi (2228.HK), the leader in the AI-pharmaceutical industry, Forcefield Ventures, and IMO Ventures, with investments from Tiantu Capital (1973.HK), Yael Capital, Fangyuan Capital, Lead Rich International, Decent Capital, and Bopu Capital.
For more information, please visit http://en.metabiopharma.com/ and follow us on LinkedIn.
Contact: contact@metabiopharma.com

On March 2-3, the two-day 2023 China Innovation and Entrepreneurship Investment Conference achievement release ceremony and the Greater Bay Area Science and Technology Conference were successfully held at the Shenzhen Futian Convention and Exhibition Center.

The conference, themed “Smart Innovations in China: Illuminating Dreams,” brought together more than 120 experts, scholars, industry leaders, representatives from 9+2 cities, and investment institutions from various fields. Through a combination of online and offline interactions, they explored cutting-edge academic achievements and development trends in areas such as sensors, nanotechnology, artificial intelligence, healthcare monitoring, and AI robotics. This event injected new energy into industrial upgrading and economic growth.
Certainly! The translation of the provided text to English is as follows:
“Wang Jinzhan, Member of the Party Group and Secretary of the Secretariat of the China Association for Science and Technology (CAST); Cheng Hongbo, Secretary of the Party Group and Vice Chairman of the Guangdong Association for Science and Technology (GAST); and Wang Qiang, Member of the Standing Committee of the Shenzhen Municipal Party Committee and Minister of the United Front Work Department, attended the main forum and delivered speeches.
Also present at the event were Song Jun, Vice Chairman of the China Technology Development Foundation, former Member of the Party Group and Secretary of the Secretariat of CAST; Zhang Qing, Director of the Enterprise Innovation Service Center of CAST; Gao Chunbo, Deputy Director of the Enterprise Innovation Service Center of CAST; Hua Xuchu, Member of the Party Group of GAST; Liao Junwen, President of the Shenzhen Association for Science and Technology (SAST), Special Invited Vice Chairman of the China Industry-University-Research Cooperation Promotion Association, Vice Chairman of the 4th Shenzhen Municipal Committee of the Chinese People’s Political Consultative Conference (CPPCC), and former Minister of the United Front Work Department; and Lin Xiang, Secretary of the Party Group and Deputy Chairman of SAST. These leaders and experts attended the event.

META stood out among several high-quality projects at this conference and was awarded the ‘Top 100’ by the conference organizing committee. This recognition reflects the acknowledgment from numerous industry experts, judges, and professional institutions, highlighting MetaNovas Biotech’s immense development potential.

On December 28, 2023, the 13th CCTV Financial Forum in Hong Kong, with the theme of ‘Technology Innovation: Activating New Drivers for Economic Development’, was held in Hong Kong. The forum aimed to facilitate discussions and exchanges on how technology innovation can empower high-quality economic development.

Hong Kong Special Administrative Region Chief Executive John Lee, National Committee Member of the Chinese People’s Political Consultative Conference (CPPCC) and Chairman and Chief Editor of Hong Kong Ta Kung Wen Wei Media Group, Lee Tai-hung, and Peng Jianming, Member of the Editorial Board Meeting and General Manager of the Central Radio and Television Station, were among the participants in the forum and delivered speeches.

Dr. Ke Xu, Co-founder and CEO of META Pharmaceuticals, was also invited to participate in the forum. During the dialogue session, Dr. Xu shared META’s achievements in utilizing artificial intelligence for biomedical research and development. He explained that META is a company focused on developing original drugs for autoimmune diseases, metabolic disorders, and rare diseases. Dr. Xu highlighted that the company has made breakthroughs in chronic disease treatment by regulating the metabolic vitality of immune cells to achieve immune system function modulation.

Dr. Ke Xu, in an interview after the forum, emphasized that the company’s use of AI and machine learning technologies can significantly enhance the efficiency of drug discovery and accelerate the progression of drugs into clinical trials. He also expressed the hope for better integration of innovative AI applications, enabling META to contribute to First-in-class original drugs and continue exploring and benefiting patients in the field of biomedicine.


On December 28, the Sixth Greater Bay Area Biotechnology Innovation Competition awards ceremony for the Top 50 Innovative Biotechnology Enterprises was held with great fanfare in Nansha, Guangzhou. The event was organized by the China Innovation Industry Research Institute, hosted by Guangdong Medical Valley, and co-organized by Haitong Securities. Simultaneously, a Biotechnology Innovation Development Summit was held, aiming to bring together experts and investment professionals from the biopharmaceutical industry, discover outstanding players in the biotechnology sector, and explore collaborative innovation for industry development.

Shu Yuan, Dean of the China Innovation Industry Research Institute; Yan Zhen, Member of the Party Group and Deputy Director of the Guangdong Provincial Drug Administration; Sheng Nanfang, former Director of the China Southern Talent Market and former Vice President of the Guangdong Talent Exchange Association; Tan Hong, Chief Economist of the Guangzhou Development and Reform Commission; Xie Wei, Deputy Secretary of the Party Working Committee of Nansha Development Zone in Guangzhou; Wang Houhua, Deputy Director of the Guangdong Productivity Promotion Center; and Professor Lin Xinhua, Executive Dean of the Precision Medicine Research Institute of the Greater Bay Area, were among the experts who attended the ceremony.
After visits and screenings by the judging panel and investors, META successfully made it to the Top 50 Innovative Biotechnology Enterprises list in the Greater Bay Area and was awarded the title of Emerging Enterprise. This recognition reflects the high regard from numerous professionals for META’s achievements.







